|
.jpg) |
|
FAQ's for Chemicals
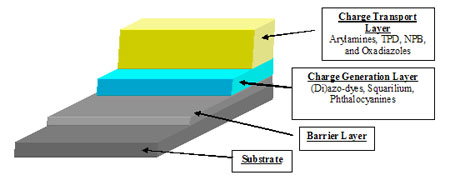
What materials are used for Organic Photoconductors?
Hole Transport Materials – Polyarylamine type structures with high charge mobility and excellent blending properties
Back to top Why are photochromic colors darker on some days than others?Photochromic colors are mostly affected by two factors: Back to top Why is there a residual color when the photochromic transition should be clear?There will be a slight residual color with photochromic transitions unless:
Back to top What should the photochromic be protected from?The photochromic molecule needs to be protected from free radicals such as singlet oxygen, oxidizers such as peroxides, acids and high energy ultraviolet, UVB. While it is robust in the closed or colorless form, it is most susceptible to attack in its open or colored state.
Back to top What is absorptivity?Absorptivity is a measure of the amount of light absorbed by a solution. Usually defined by Beer’s Law, absorptivity is proportional to the concentration of the absorbing solute. molar absorptivity is absorptivity defined in terms of concentrations expressed in moles per liter. Symbol e. e=A/cl c=M(moles/liter) e has the units of L mol-1 cm-1 specific absorptivity is absorptivity defined in terms of concentrations expressed in grams per liter. Symbol a. a =A/cl c=grams/liter Back to top How long will photochromic features last?Photochromics that form acidic compounds when exposed to sunlight will not last as long in certain materials. In plastisol, the half life of these pigments have achieved greater than 40 hours in a QUV panel. (The half life is the time it takes to reach one half the initial change in color intensity). And with products in solvent cast urethane, they have shown a half life greater than 2,000 hours.
Back to top Can you help me find a compound by its common name?Yes, our staff has extensive knowledge of chemical structures, compounds, and nomenclatures.
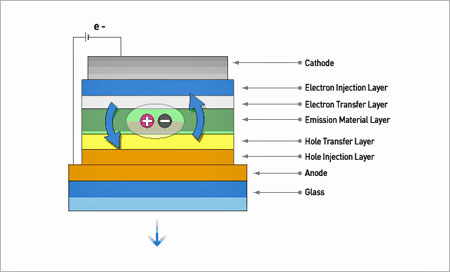
Back to top What is the structure of a typical OLED?
• Mechanism of OLED Device
Back to top
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
Terms and Conditions | Site Map